According to the researchers, the findings offer new insights into U(VI) reduction by sulfate-reducing bacteria and contribute to a comprehensive safety concept for a deep geologic repository for high-level radioactive waste. Their findings have been published in the journal Science of the Total Environment.
The rock: Clay rock is considered a favorable host rock for HLW geologic repositories because of its low permeability and ability to retain radionuclides. Bentonite, which consists of various clay minerals, can be further used to backfill waste packages as an engineered barrier.
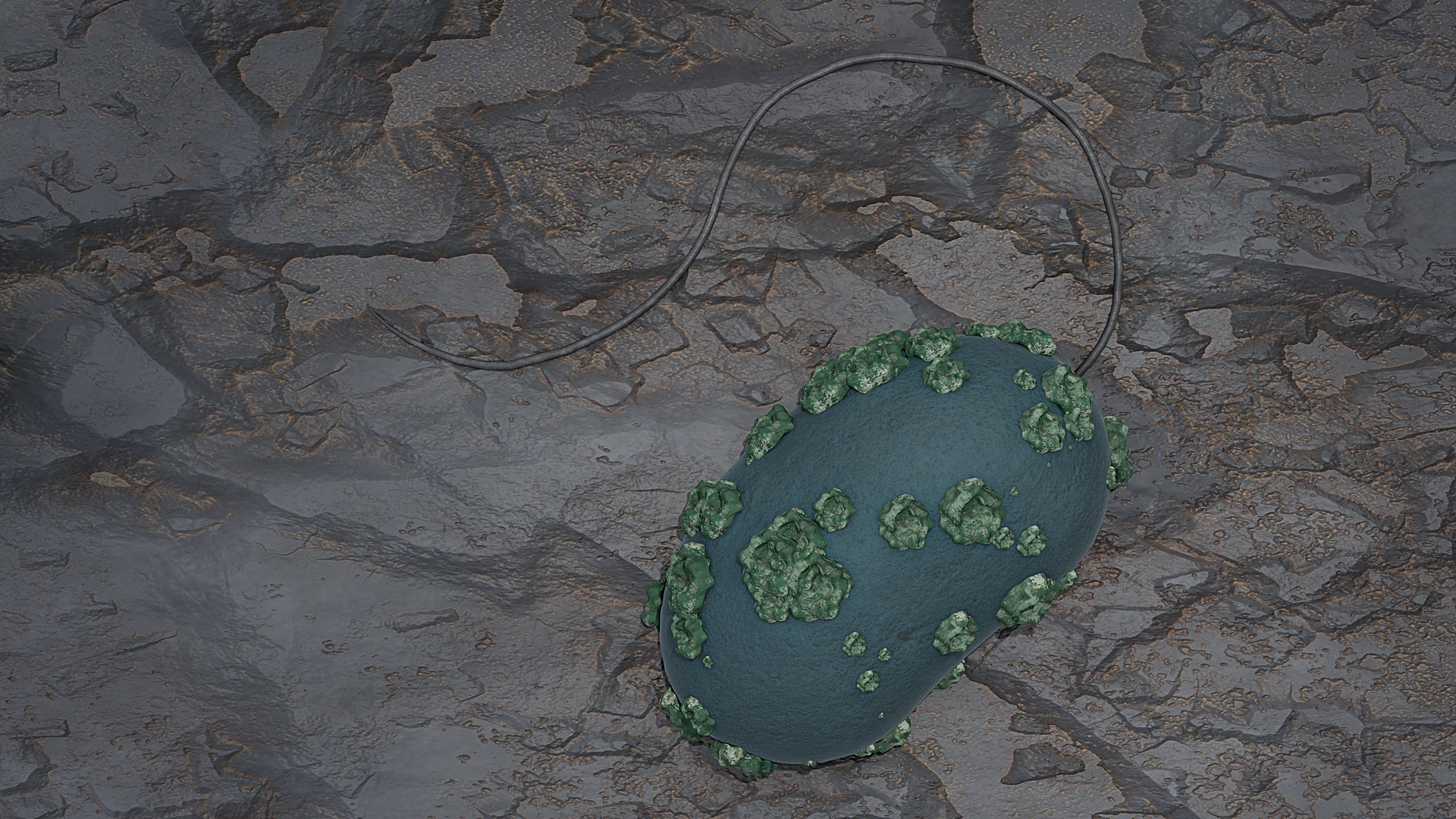
“We know that so-called sulphate-reducing microorganisms occur in both the host rock and in the backfill material,” said Stephan Hilpmann, of the HZDR Institute of Resource Ecology. “In our work, we investigated a representative of the genus Desulfosporosinus in more detail. We were particularly interested in its influence on uranium present in the bentonite-clay system.”
The study: As Desulfosporosinus bacteria live under anaerobic conditions, researchers were able to study the microorganism under realistic conditions, such as those found in deep layers of rock. To do this, they brought bacterial cultures into contact with uranium salt solutions in natural pore water of the clay rock, covered by a nitrogen atmosphere that protects them from atmospheric oxygen.
They observed that the bacteria convert the easily water-soluble U(VI) into sparingly soluble U(IV). The bacteria can deposit u(IV) in membrane vesicles on their cell surfaces in the form of incrustations. The team assumes that this is a defensive reaction of the microorganisms—a behavior that has previously been observed in other types of bacteria.
“After one week, the bacteria have converted about 40 percent of the originally dissolved uranium into the poorly soluble variant,” Hilpmann noted.
The team also observed a further oxidation stage with U(V), mainly due to its typical instability. They suspect that they were only able to detect U(V) because the bacteria stabilized it to some extent in solution. This oxidation state was detectable even after one week.
The multispectral view: To observe the various uranium compounds, the team used a range of modern spectroscopy and microscopy methods. The HZDR researchers have access to specialized techniques at the Institute of Ion Beam Physics and Materials Research and at the Rossendorf Beamline, which the HZDR operates at the European Synchrotron Radiation Facility in Grenoble, France.
At the French site, for example, they can investigate radiochemical processes spectroscopically. Here they have also observed the formation of U(V) in the process using a method called HERFD-XANES.
HERFD-XANES stands for fluorescence detection with high-energy resolution, which is coupled with X-ray near-edge absorption spectroscopy. This is an X-ray absorption spectroscopic method that can be used to study the behavior of electrons. The team was able to visualize the uranium-containing aggregates on the cell surface of Desulfosporosinus using scanning transmission electron microscopy coupled with energy-dispersive X-ray spectroscopy.
“Our findings deepen our understanding of the complex processes in a potential final repository,” Hilpmann said. “They may also be relevant for the removal of radioactive pollutants from contaminated waters and thus for their remediation.”