For good reason: Uranyl fluoride, a molecule containing uranium, oxygen, and fluorine, can indicate that a sample of uranium has been enriched. “Being able to determine how much fluorine versus uranium is present can give you additional information about where that particle came from, what processes produced that particle, and how long ago those processes occurred,” explained Brian Ticknor, a coauthor of the study.
ORNL’s Benjamin Manard led the study, which was funded by ORNL’s Laboratory Directed Research and Development program. Manard developed experiments for a multidisciplinary team at ORNL’s Ultra-trace Forensic Science Center, which in part supports the IAEA with advanced characterization of nuclear materials and novel fuel cycle processes.
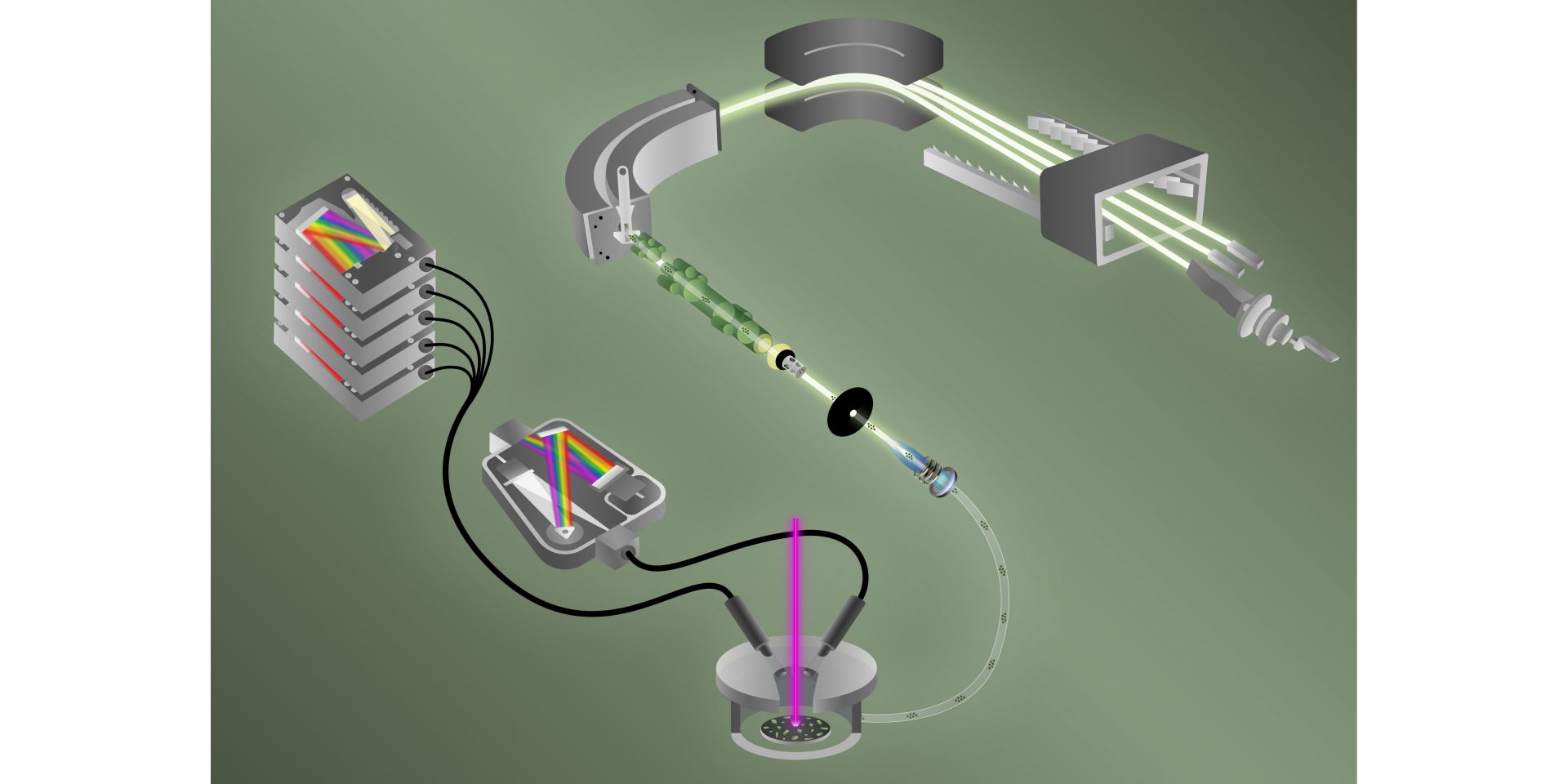
How it works: While detection of fluorine and of uranium isotopes is not new, pairing the two for simultaneous testing is. An animation produced by ORNL shows how the two techniques work in tandem, starting with a high-energy pulsed laser.
Within the new detector, laser-induced breakdown spectroscopy, or LIBS, tackles fluorine.
“LIBS vaporizes a sample, like a uranyl fluoride particle, breaking it down and forming a plasma, or cloud of excited ions. As the plasma cools, light is emitted,” said ORNL’s Hunter Andrews, the study’s LIBS task lead. Spectroscopy then measures the light to characterize elements in the plasma.
Simultaneously, helium gas sweeps atoms of the plasma into a mass spectrometer, where isotopes of uranium are characterized via the second technique, called laser ablation multicollector inductively coupled plasma mass spectrometry, or ICP-MS.
“LIBS tells us if and how much fluorine is in the particle, while ICP-MS tells us all of the uranium isotopes present,” Manard said. “This integrated equipment is a one-stop shop to measure fluorine as well as uranium isotopes at the same time.”
While ICP-MS needs a positive charge to make a measurement, “elements like fluorine are not amenable to the ICP-MS that we do for uranium,” Ticknor said. “It has to do with the electronegativity of fluorine. With ICP, the goal is to form positive ions to inject into the mass spectrometer. Fluorine very, very strongly wants to have a negative charge.” Adding LIBS allows for the analysis of negative fluorine ions.
The results: “Determining isotopic ratios on single particles takes a lot of time,” said Manard. “Rapid particle analysis for fluorine and uranium isotopic determination is what we've enabled.” The team’s detector can analyze 40 particles—each about the size of a red blood cell—in less than five minutes, according to ORNL.
“My research has recently centered around high-throughput particle analysis,” Manard added. “Due to improvements in laser ablation, we've been able to push the limits of how fast we can characterize single particles. We're trying to push through thousands of particles in 24 hours with this technology. Speed is always in the back of my mind.”
More applications: Manard and Ticknor envision applications for their technique beyond nuclear nonproliferation. The two techniques could reveal the origin and evolution of isotopes in other fields—including geology, biology, and chemistry—and could potentially be extended to other challenging compounds relevant to nuclear processes, according to ORNL.
“Chlorine is right below fluorine on the periodic table and has a lot of the same properties,” Ticknor said. “Uranium chloride has been hard to measure in the past, and it would potentially be amenable to this type of measurement.”