Henri Becquerel and the Discovery of Radioactivity
X-Rays
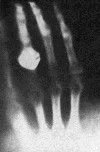
In January 1896, Wilhelm Röntgen astonished the world by circulating photographs of the bones in his hand, taken with the aid of his new discovery: X-rays. The photographs of the interior of the human body caused a worldwide sensation that year. It also stirred Antoine Henri Becquerel to action. Becquerel thought that the X-rays were coming from a region of Röntgen's vacuum tube made phosphorescent by the cathode rays traveling through it, and as a world expert in phosphorescence, he immediately committed to find out whether all phosphorescent material produced these extraordinary X-rays.
Phosphorescence
 First, let us illuminate the subject of phosphorescence, which was mysterious and a hot topic of research in the late 1800s. While luminescence is the emission of light by a substance not resulting from heat, phosphorescense is the special case of a material emitting light for some period of time after it has absorbed photons. It would take decades of research and the theory of quantum mechanics to construct a model of how a material could "store" energy and then slowly emit it in the form of light long after the source of energy had been removed.
First, let us illuminate the subject of phosphorescence, which was mysterious and a hot topic of research in the late 1800s. While luminescence is the emission of light by a substance not resulting from heat, phosphorescense is the special case of a material emitting light for some period of time after it has absorbed photons. It would take decades of research and the theory of quantum mechanics to construct a model of how a material could "store" energy and then slowly emit it in the form of light long after the source of energy had been removed.
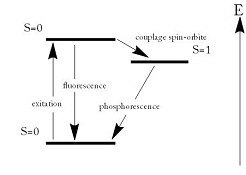 Normal fluorescence involves, roughly, an electron of an atom being excited to a higher energy state or "orbital"-by absorbing a photon of energy-and then returning to its ground state, releasing a photon of lower wavelength, almost instantaneously. But in the special case of phosphorescence in some materials, an electron can move to a so-called "forbidden" higher energy state by way of absorption of a photon of energy-and the process of releasing the energy from such a forbidden state occurs more slowly, overall, than the straightforward case of fluorescence-resulting in a material "glowing" for longer periods after exposure to photons.
Normal fluorescence involves, roughly, an electron of an atom being excited to a higher energy state or "orbital"-by absorbing a photon of energy-and then returning to its ground state, releasing a photon of lower wavelength, almost instantaneously. But in the special case of phosphorescence in some materials, an electron can move to a so-called "forbidden" higher energy state by way of absorption of a photon of energy-and the process of releasing the energy from such a forbidden state occurs more slowly, overall, than the straightforward case of fluorescence-resulting in a material "glowing" for longer periods after exposure to photons.
Not phosphorescence
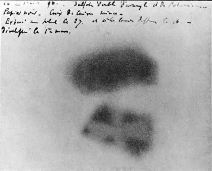
Unaware of any of this, Becquerel nonetheless placed his potassium uranyl sulfate K2UO2(SO4)2 compound onto photographic plates, covered the plates with black paper to protect them from light, and put them on the windowsill so that the uranium compound could absorb light and begin to glow. As he expected, the rays from the phosphorescing uranyl sulfate penetrated the paper and exposed the photograph, and even penetrated metallic objects as well. Perhaps not as clearly as Röntgen's vacuum tube X-rays would have done, but a marvelous result nonetheless.
Then something unexpected and amazing happened. Becquerel was ready to perform more windowsill experiments, but cloudy days ensued and so he placed the plates and minerals inside of a dark desk drawer. No light absorption or phosphorescence was occurring in there, and simply on a hunch (or more probably, he was just being thorough), Becquerel developed these plates anyway, expecting very faint or no image at all. Instead, he found intense images-in effect, the uranium was emitting X-rays all on its own. To the scientists of the day, this was astounding-energy being created out of "nothing"-except that the experiment was easy for them to duplicate to duplicate Becquerel's findings. Becquerel also quickly found that the effect did not diminish with time-the uranium disulfate continued to constantly emit energy-and pure metallic uranium worked even better.
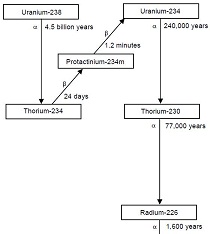 We now know that the uranium was emitting a wide range of energy besides X-rays, in a spontaneous process soon to become known as radioactivity. We also know that the uranium was spontaneously decaying to a lower energy isotope while emitting alpha particles, with "daughter nuclides" emitting beta particles. Becquerel, as well as Marie and Pierre Curie, were instrumental in researching this new and incredible property of matter called radioactivity, and all three shared the Nobel Prize in physics in 1903. And, while it is often stated in games of chance, "I'd rather be lucky than good," Becquerel was both lucky and good-his lifetime of preparation in the study of phosphorescence and phosphorescent materials, his expertise in the scientific method and in laboratory uses of photography, and a great innate curiosity equipped him to make one of the more amazing discoveries in the history of science.
We now know that the uranium was emitting a wide range of energy besides X-rays, in a spontaneous process soon to become known as radioactivity. We also know that the uranium was spontaneously decaying to a lower energy isotope while emitting alpha particles, with "daughter nuclides" emitting beta particles. Becquerel, as well as Marie and Pierre Curie, were instrumental in researching this new and incredible property of matter called radioactivity, and all three shared the Nobel Prize in physics in 1903. And, while it is often stated in games of chance, "I'd rather be lucky than good," Becquerel was both lucky and good-his lifetime of preparation in the study of phosphorescence and phosphorescent materials, his expertise in the scientific method and in laboratory uses of photography, and a great innate curiosity equipped him to make one of the more amazing discoveries in the history of science.
Afternote: Becquerel (Bq)
The Becquerel (Bq) is the international unit of radioactivity, named after our pioneer Henri Becquerel. It is defined as one atomic nucleus in a material decaying per second.
_________________
Paul Bowersox's love for nuclear history will never decay. He is a regular contributor to the Nuclear Cafe!