A vial of Ac-225 produced by Niowave stands next to its lead shipping pig. (Photo: Niowave)
According to the Council on Radionuclides and Radiopharmaceuticals, more than 82,000 nuclear imaging procedures using nuclear medicine are performed throughout the world every day. To administer these vital medical procedures, radiopharmaceutical companies and hospitals rely on a handful of producers of medical radioisotopes.
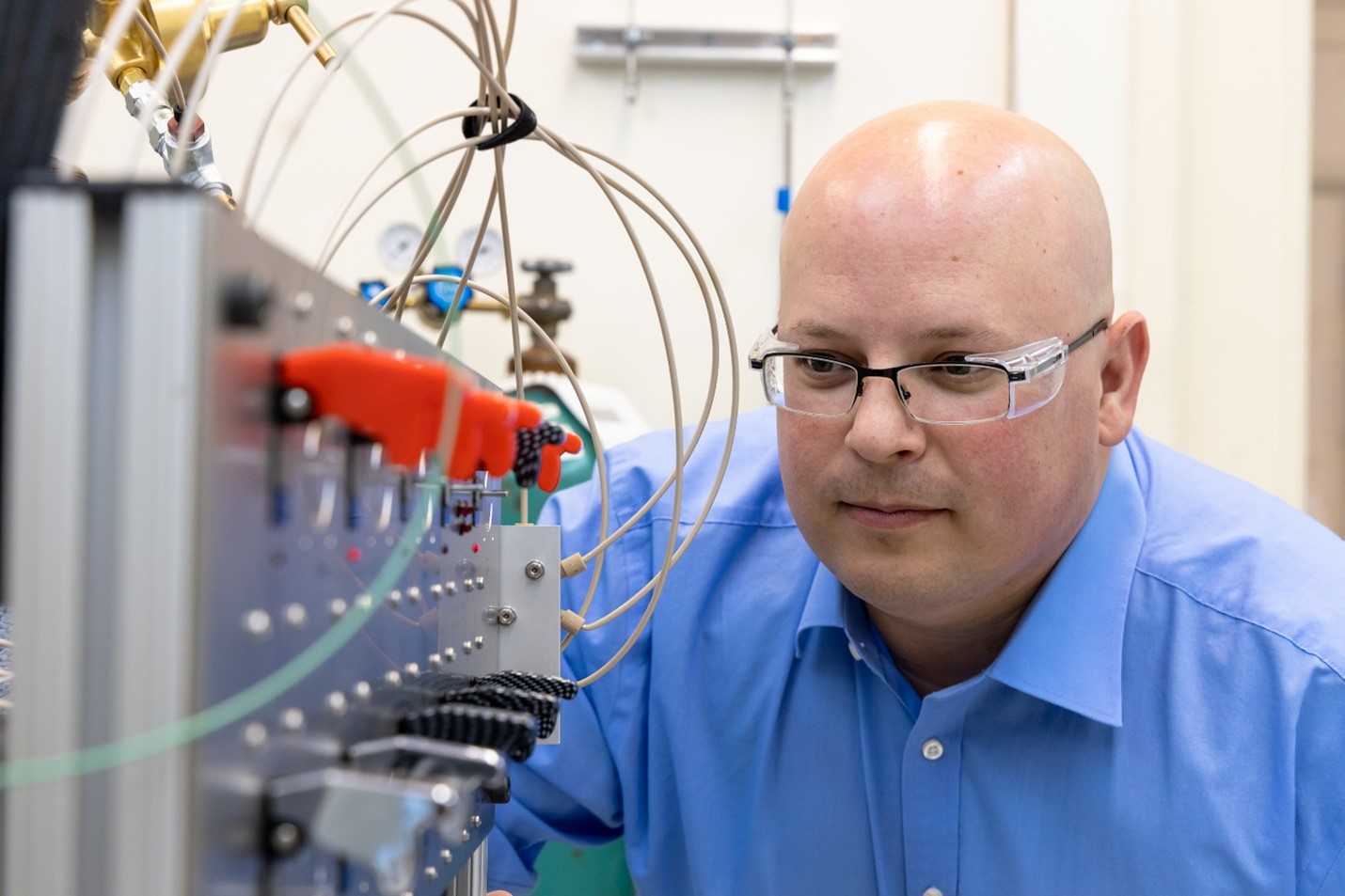
Chemist Kevin Gaddis has adapted components of a high-pressure ion chromatography system to withstand the extreme conditions of a hot cell. (Photo: ORNL/Carlos Jones)
An Oak Ridge National Laboratory researcher has built a device that can speed up the separation of the medical radioisotope actinium-225 from irradiated thorium targets and withstand the high-radiation environment of a hot cell. In July, ORNL announced that Kevin Gaddis, a chemistry technician at the lab, had built and tested a prototype and was working to secure a patent for a device that cut separations time by 75 percent.



 QSA Global, a provider of radioisotope products, and Niowave, a Michigan-based producer of medical radioisotopes, announced that the companies will codevelop a scalable radium purification process using Niowave’s radium-226 processing technology to meet the demand for actinium-225, an alpha-emitter used in the treatment of cancer. According to the companies, the strategic partnership marks a significant advancement in the field of radiopharmaceutical technology, enhancing the supply chain for critical radioisotopes, including Ac-225.
QSA Global, a provider of radioisotope products, and Niowave, a Michigan-based producer of medical radioisotopes, announced that the companies will codevelop a scalable radium purification process using Niowave’s radium-226 processing technology to meet the demand for actinium-225, an alpha-emitter used in the treatment of cancer. According to the companies, the strategic partnership marks a significant advancement in the field of radiopharmaceutical technology, enhancing the supply chain for critical radioisotopes, including Ac-225.